Select the Statement That Best Describes a Buffer S
Log in to add comment. 11218 958 PM MB Chp.
Solved Part A Select The Statement That Best Describes A Chegg Com
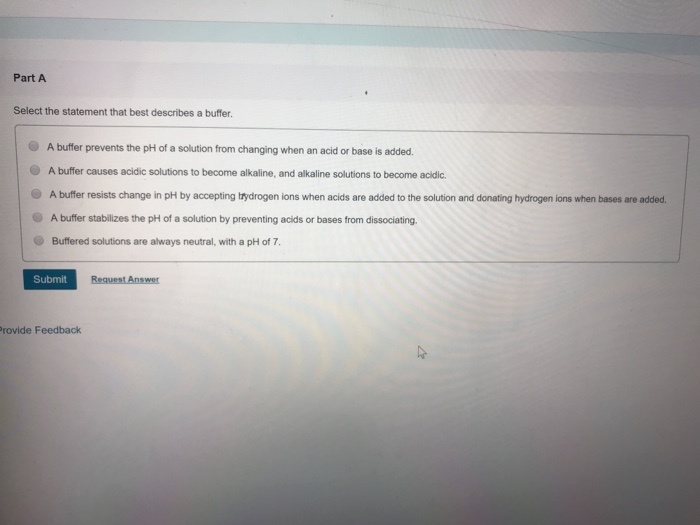
B A buffer prevents the pH of a solution from changing when an acid or base is added.

. - A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. Part A Select the statement that best describes a buffer. Select the statement that best describes a chemical buffer.
B A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. View Available Hints Select the statement that best describes a buffer. Select the statements that correctly describe buffers 厂 The pH of a buffer solutions determined by the ratio of the concentration of con gate base to the concentration of strong acid.
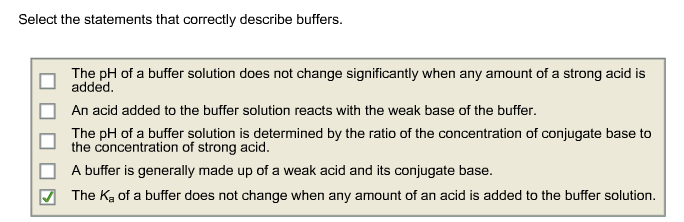
1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added. O A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. The pH of a buffer solution does not change significantly when a small amount of acid is added.
O a substance that minimizes changes in pH O a substance that raises pH O a substance that completely dissociates in water O a substance that is a mixture of water and an acid. 2 The Ka of a buffer does not change when any amount of. The pH of a buffer solution does not change significantly when any amount of a strong acid is added.
A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. A buffer prevents the pH of a solution from changing when an acid or base is added. A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic.
100 39 ratings Transcribed image text. Buffered solutions are always neutral with a pH of 7. Buffer resists change in pH by accepting ttydrogen ions when acids are added to the solution and donating.
A buffer prevents the pH of a solution from changing when an acid or base is. Select the statement that best describes a buffer. Added An acid added to the buffer solution reacts with the weak base of the buffer.
A buffer causes acidic solutions to become. Select the statement that best describes a chemical buffer. Concept 33 A buffer accepts hydrogen ions when they are in excess and donates hydrogen ions when they have been depleted.
- A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. A substance that resists small change in the acidity of a solution when an acid or base is added to the solution. A A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added.
A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. 3 The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of.
Up to 256 cash back Select the statement that best describes a buffer. O A buffer prevents the pH of a solution from changing when an acid or base is added. The pH of a buffer solution is determined by the ratio of the concentration of acid to the concentration of base.
A chemical substance that prevents changes in hydrogen ion concentration. An acid added to the buffer solution reacts with the weak base of the buffer. Which statement best describes how buffers perform their function.
Select the statement that best describes a buffer. Select the statements that correctly describe buffers. 1 The pH of a buffer.
Select the statement that best describes a buffer. A buffer prevents the pH of a solution from changing when an acid or base is added. An acid added to the buffer solution reacts with the weak base of the buffer.
1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added. Select the statement that best describes a buffer. A buffer is generally made up of a weak acid and its conjugate base.
A buffer stabilizes the pH of a. AA buffer prevents the pH of a solution from changing when an acid or base is added. A Select the statement that best describes a buffer.
BA buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. A buffer is generally made up of a weak acid and its conjugate base. Select the statements that correctly describe buffers.
2 The Ka of a buffer does not change when any amount of an acid is added to the buffer solution. 3 Flashcards Quizlet Select the statement that best describes a buffer. The K_a of a buffer does not change when any amount of an acid is added to the buffer solution.
DA buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating. Select the statements that correctly describe buffers. A buffer is made up of a strong acid and a strong base.
AA buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. The pH of a buffer solution is determined by the ratio of the. Select the statements that correctly describe buffers.
A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. Which of the following sentence best describes a buffer. O Abuffer resists change in pH by accepting ttydrogen ions when acids are added to the solution and donating hydrogen ions.
Select the statements that correctly describe buffers. O a substance that minimizes changes in pH O a substance that raises pH O a substance that completely dissociates in water O a substance that is a mixture of water and an acid.
Ccna Security V2 0 Chapter 5 Answers Implementing Network Security

Solved Short Answer Write The Word Or Phrase That Best Chegg Com
Solved Select The Statements That Correctly Describe Chegg Com
Comments
Post a Comment